
Streamline critical document workflows and ensure compliance with Nutrient’s automated workflow solutions tailored to the pharmaceutical industry.
USE CASES
Support secure, compliant collaboration from research through production with workflows that simplify regulatory and quality processes.

Automate trial documentation and approvals, ensuring timely and accurate reporting.

Streamline the preparation and submission of regulatory documents, reducing errors and speeding up the approval process.
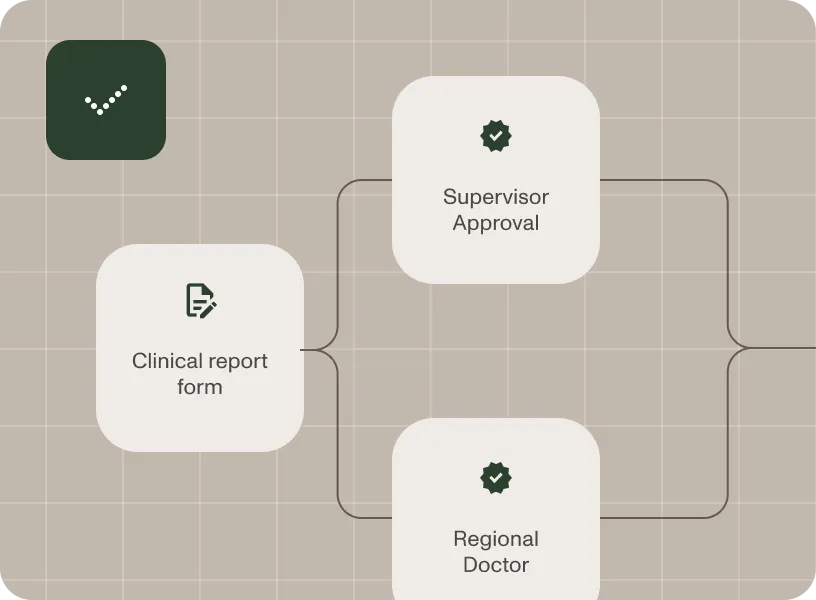
Automate quality management workflows to ensure consistent product quality and compliance with good manufacturing practice (GMP) standards.

Enhance collaboration between research teams with automated document sharing and approval processes.
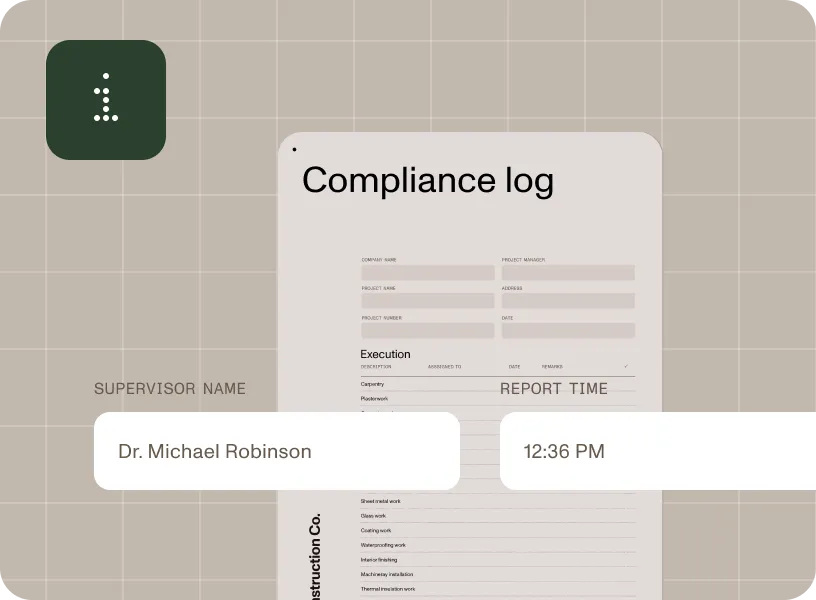
Simplify the tracking and documentation of manufacturing processes to maintain compliance with industry regulations.
PROVEN AT SCALE
Digitized global CapEx approvals with Nutrient Workflow’s cloud solution — ensuring full audit compliance, real-time reporting, and mobile approvals across sites.
Standardized global IT request management with Nutrient Workflow — processing 16,000+ requests per month across 60 countries. Cut costs by up to 90 percent per transaction and simplified user requests with one universal interface.
Automated new hire onboarding, cutting cycle times by 3–5 days. Now runs 25 processes across HR and IT, handling 400–500 monthly requests from 900 employees across 170+ clinics — with full visibility and centralized control.
Workflow Automation integrates with your tech stack — including finance systems, procurement platforms, and approval tools — using APIs, webhooks, or SFTP. No extra middleware required.
Contact us today to find out how workflow management can help pharmaceutical and biotech companies.
Nutrient’s platform is designed to optimize and automate document workflows in the pharmaceutical sector, enhancing efficiency, ensuring compliance, and accelerating time to market for new products.
The platform supports automation of various processes, including:
By automating regulatory processes, the platform ensures consistent adherence to industry standards, reduces the risk of non-compliance, and maintains accurate, up-to-date records throughout the product lifecycle.
Yes. It enhances collaboration by automating document sharing and approval processes, facilitating seamless communication between research teams, and expediting project timelines.
Yes. Nutrient’s platform is flexible and scalable. It’s capable of accommodating the unique operational requirements of various pharmaceutical projects, providing a better experience for teams and stakeholders.